






Lactitol is a 12-carbon sugar alcohol, which can be obtained by catalytic hydrogenation of lactose. There are anhydrous and two products containing a bound water. It is sweet and refreshing, often used in combination with high-intensity sweeteners, without aftertaste and hygroscopic Its relative molecular mass is similar to that of sucrose, and its influence on water activity is similar to that of sucrose. It is stable under acidic and alkaline conditions, and is also very stable under high temperature conditions of food processing. Lactitol is used in many foods, such as baked goods, sugar-coated candies, and frozen dairy-based confections.
Appearance
Lactitol is white crystal or crystalline powder, or colorless liquid. Odorless and sweet, the sweetness is 30% to 40% of sucrose, and the calories are about half of sucrose (8.4kJ/g).
Structure
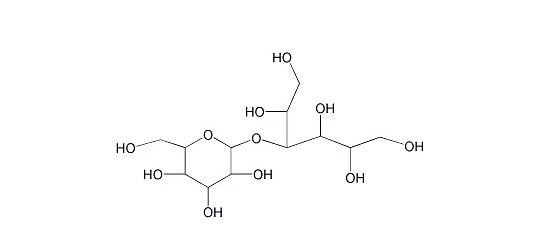
The chemical nature of lactitol is 4-O-β-D-galactopyranosyl-D-sorbitol, its molecular formula is C12H24O11, and its molecular weight is 344.32. There are two main forms of crystalline lactitol: lactitol dihydrate and lactitol monohydrate, another commercial lactitol is lactitol containing 54% lactitol solution.
Melting point
The anhydrous lactitol is 146 °C, the monohydrate is 94-97 °C, and the dihydrate is 70-80 °C. When the hydrate is heated to above 100 °C, it gradually loses water, and above 250 °C, intramolecular dehydration occurs to form milk caramel.
Solubility
Lactitol is easily soluble in water and slightly soluble in ethanol, and the pH of 10% lactitol aqueous solution is 4.5-8.5. The solubility and viscosity of lactitol at room temperature are similar to those of sucrose, and its solubility at low temperature is lower than that of sucrose. Plus, not only does lactitol not give off heat when it dissolves, it absorbs it.
Stability
Lactitol has strong stability and can still maintain its stability under acid, alkali, light and high temperature conditions. In addition, there is no free hydroxyl group in the molecular structure of lactitol, which is a non-reducing sugar alcohol, and cannot undergo Maillard reaction and enzymatic degradation reaction. In this way, adding lactitol to foods requiring high temperature treatment or acidic and alkaline foods can avoid and reduce the deterioration of foods, and better maintain the original color, aroma and taste of foods.
Manufacturing method
Lactitol is prepared from skim milk, then subjected to pressure hydrogenation (100 °C, 30%-40% lactose liquid, 4MPa) under nickel catalysis, filtered, purified by ion exchange resin and activated carbon, concentrated and crystallized.
Safety
1. ADI: no special provisions are made (JECFA2006; the ADI value was first established in 1983).
2. Mutagenicity test: micronucleus test, sperm aberration test and Ames test were all negative.
3. Large doses can cause diarrhea.
4. LD50: Mice orally more than 10g/kg (body weight, bw)
Health Care Function
Low calorie
Lactitol has a lower caloric value because its metabolic pathway is different from that of sucrose (lactitol cannot be digested and absorbed in the small intestine, but is fermented and utilized by microorganisms in the large intestine and decomposed into short-chain fatty acids and other fermentation products, which release Pure calories are only 50% of ordinary sugars. This allows lactitol alone or mixed with other sweeteners to replace sucrose low-calorie health food.
Obesity prevention
The intake of high-fat foods and sucrose will stimulate the secretion of insulin in the blood and increase the activity of ribozymes in adipose tissue, thereby promoting the accumulation of neutral fat in cells. The intake of lactitol will not cause the rise of insulin and will not increase nuclear The activity of protein lipase does not promote the accumulation of neutral fat in the body when ingested with high-fat foods. Therefore, from a preventive standpoint, in foods that are high in fat and sucrose, it is beneficial to replace sucrose with lactitol, which is low in calories and does not stimulate insulin rise.
Prevent dental caries
Lactitol has a strong function of preventing dental caries. Compared to sucrose, lactitol hardly causes dental plaque formation. Clinical experiments have confirmed that lactitol will not be fermented by microorganisms in the oral cavity to produce acids, and such acids can destroy the enamel of the teeth through deionization, resulting in dental caries. Various candies, etc.

Application
Sweetener; Emulsifier; Stabilizer; Thickener
The application of lactitol largely lies in the replacement of sucrose. Products using this sweetener can be used as low-calorie, sugar-free, caries-free and suitable for diabetics. Lactitol is used in the production of a wide variety of confectionery, such as chocolate, hard candy, caramel, soft candy and chewing gum, as well as ice cream and baked goods. All of these products demonstrate the versatility of lactitol as a bulk sweetener.
In the production of chewing gum, lactitol can replace sorbitol to avoid moisture absorption during production and storage. This means that cheaper packaging materials can be used, which also has a positive impact on the environment. In hard and soft candies, the mixture of lactitol and maltitol syrup is similar to traditional confectionery products in terms of processing and quality of the final product.
In addition, lactitol can be used as a quality improver in livestock feed to improve animal liking, and can also promote the proliferation of bifidobacteria in animals. It can be used as an excipient for preparations in medicine, and it is also used in cosmetics such as toothpaste, cigarettes, and lipstick. With the diversification of food and the continuous improvement of the comprehensive quality of color, aroma and taste, lactitol as a new type of functional sweetener has received more and more attention and development.
